Introduction
Effective symptom management is crucial in palliative care, and routine symptom assessment forms its foundation. Among the various tools available, the Edmonton Symptom Assessment System (ESAS) stands out as a pioneering quantitative measure. This simple yet rapid tool allows healthcare professionals to document multiple patient-reported symptoms simultaneously, providing a comprehensive overview of a patient’s symptom burden. Since its inception, ESAS has become widely recognized and utilized across different medical settings. This article delves into What Is The Esas Tool Palliative Care, exploring its historical development, diverse applications in contemporary healthcare, and potential future advancements. We aim to provide a comprehensive understanding of ESAS and its significance in enhancing palliative care practices and research.
Understanding the ESAS Tool: Development and Evolution
Origins of ESAS
The ESAS was initially conceived in 1991 by Dr. Eduardo Bruera and his colleagues as a practical clinical instrument. Its primary purpose was to systematically document the range of symptoms experienced by patients with advanced cancer who were admitted to a palliative care unit. The original ESAS comprised eight horizontal 0–100 mm visual analog scales (VAS). These scales were designed to assess the intensity of common symptoms such as pain, activity levels, nausea, depression, anxiety, drowsiness, appetite, and a general sense of well-being. Additionally, a ninth VAS was included to capture any other less frequent, yet significant symptom specific to an individual patient’s condition. Patients, along with their relatives or nurses, were instructed to complete the ESAS twice daily. The focus of this initial version was to evaluate the current intensity of symptoms at the time of assessment. To quantify overall symptom distress, the researchers introduced a symptom distress score, calculated by summing the scores of the initial eight symptoms. This early application of ESAS in a palliative care setting quickly demonstrated its utility as a straightforward and effective method for regularly assessing symptom distress.
Validation and Modifications Over Time
Since its initial development, the ESAS tool has undergone rigorous validation and refinement by numerous research teams worldwide, solidifying its position as a reliable instrument in palliative care. In 1993, Bruera et al. conducted a study that confirmed the ESAS’s strong test-retest reliability among hospitalized patients. They also found significant correlations with the Support Team Assessment Schedule (STAS), further establishing its validity. Philip and colleagues evaluated a slightly adapted version of ESAS, focusing on symptoms “now” and replacing “activity” with “weakness,” and observed satisfactory correlations with other established symptom assessment tools like the Brief Pain Inventory and the Rotterdam Symptom Checklist. Chang et al.’s prospective study involving a larger cohort of cancer patients in the US further supported the ESAS’s psychometric properties, demonstrating good internal reliability, test-retest reliability, and convergent validity when compared to other pain assessment scales. These comprehensive validation studies have been extensively reviewed, underscoring the ESAS’s robust psychometric foundation. More recently, studies have also highlighted the predictive validity of ESAS, with higher symptom burden scores being associated with increased emergency room visits and reduced survival times.
Over the years, practical modifications have been made to enhance the usability and patient understanding of the ESAS tool. The original VAS format was transitioned to 11-point numeric rating scales (NRS), ranging from 0 (no symptom) to 10 (worst possible). This shift to NRS was driven by its simplicity and ease of use for patients, while maintaining comparable data quality to VAS. The symptom items themselves have also been refined. “Activity” was replaced with “tiredness/fatigue,” and “shortness of breath” was added as a core symptom. Furthermore, additional symptoms such as “constipation,” “insomnia,” “spiritual distress,” and “financial distress” have been suggested for inclusion to broaden the tool’s applicability. For daily use, the timeframe for assessment was adjusted to capture the average symptom intensity over the past 24 hours, rather than just “now,” to better reflect the fluctuating nature of many symptoms.
Patient feedback has played a crucial role in these modifications. Studies exploring patient perceptions of ESAS revealed instances of misinterpretation, particularly with items like appetite and sleep, leading to scoring errors. A “think-aloud” study by Watanabe et al. highlighted difficulties in understanding terms like depression, anxiety, appetite, and well-being, as well as challenges in distinguishing between tiredness and drowsiness. These insights led to the development of the revised ESAS numeric rating scale (ESAS-r), featuring nine core symptoms and an optional tenth. ESAS-r clarified the time frame to “now,” provided brief explanations for potentially ambiguous terms like tiredness, drowsiness, depression, anxiety, and well-being, changed “appetite” to “lack of appetite,” reorganized symptom order, improved readability, and suggested constipation as a potential tenth item. Comparative studies have demonstrated that ESAS-r is easier for patients to understand. Further refinement led to the ESAS-r-CS, which incorporated constipation and sleep and was also found to be more understandable, although patient preference for the “24-hour” timeframe remained significant. The Supportive Care team at MD Anderson Cancer Center utilizes a version (Figure 1) which includes 10 items, replaces “other symptom” with “sleep,” and assesses symptom intensity over the past 24 hours, representing the ongoing evolution of ESAS in clinical practice.
Figure 1. Edmonton Symptom Assessment Scale.
The Edmonton Symptom Assessment Scale, a crucial palliative care ESAS tool, currently used at MD Anderson Cancer Center, employs a 24-hour timeframe for assessing symptom intensity on a 0–10 numeric rating scale, aiding in comprehensive symptom management.
Translation and Cultural Adaptation
A key factor in the global adoption of ESAS as a palliative care tool is its extensive translation and cultural adaptation. Professionally translated by Mapi Research Trust into over 20 languages, ESAS is freely accessible, ensuring its availability to diverse patient populations worldwide. Beyond linguistic translation, rigorous linguistic and psychometric validation has been conducted in numerous languages, including Chinese, Flemish, French, German, Icelandic, Italian, Japanese, Korean, Portuguese, Spanish, Thai, and Turkish. This comprehensive validation process ensures that the ESAS tool maintains its reliability and validity across different cultural contexts, making it a truly universal instrument for symptom assessment in palliative care. The availability of ESAS in multiple languages (Table 1) underscores its commitment to reaching and effectively serving diverse patient communities globally.
Table 1. Language Availability for the Edmonton Symptom Assessment System
| Country | Language | Psychometrically validated in language (reference) | Linguistically validated by Mapi Research Institute |
|---|---|---|---|
| Argentina | Spanish | – | ✓ |
| Australia | English | (12) | ✓ |
| Belgium | Flemish | (27) | – |
| Brazil | Portugese | (35) | ✓ |
| China | Chinese | (26) | ✓ |
| Canada | EnglishFrench | (10, 11)- | ✓✓ |
| Denmark | Danish | – | ✓ |
| France | French | (28) | ✓ |
| Germany | German | (29) | ✓ |
| Hungary | Hungarian | – | ✓ |
| Iceland | Icelandic | (30) | – |
| Israel | HebrewRussianArabic | — | ✓✓✓ |
| Italy | Italian | (31) | ✓ |
| Japan | Japanese | (32) | ✓ |
| Korean | Korean | (33). | |
| Netherlands | Dutch | – | ✓ |
| New Zealand | English | – | ✓ |
| Portugal | Portugese | – | ✓ |
| Poland | Polish | – | ✓ |
| Russia | Russian | – | ✓ |
| Saudi Arabia | Arabic | (38) | – |
| South Africa | EnglishAfrikaans | – | ✓✓ |
| Spain | Spanish | (34) | ✓ |
| Sweden | Swedish | – | ✓ |
| Thailand | Thai | (36) | |
| Turkey | Turkish | (37) | ✓ |
| United Kingdom | English | – | ✓ |
| United States | EnglishSpanish | (13)- | ✓✓ |

The widespread availability of the palliative care ESAS tool in over 20 languages, as shown, ensures its global applicability and cultural relevance in diverse healthcare settings.
Interpreting ESAS Scores and Clinical Significance
Understanding how patients interpret the 0–10 NRS scale is essential for effective clinical application of the ESAS tool in palliative care. Researchers have investigated the cutoffs within this scale to define different levels of symptom burden: none, mild, moderate, and severe. A study by Selby et al. involving 400 cancer patients indicated that a score of 7 is generally the optimal cutoff for severe pain, depression, anxiety, drowsiness, appetite, and well-being, while 8 is suitable for severe fatigue, and 6 for dyspnea. A systematic review by Oldenmenger analyzed 18 studies and found that cutoffs for moderate symptom intensity typically range between 4 and 5, and for severe symptom burden between 7 and 8. Consistent findings were reported in a recent study on the Japanese version of ESAS-r, suggesting that these cutoffs are relatively consistent across different cultures and patient populations. In clinical practice, scores of 0, 1–3, 4–6, and 7–10 on the ESAS are generally interpreted as none, mild, moderate, and severe symptom intensity, respectively. However, it is crucial to recognize that individual patient interpretation of these scores can vary significantly, emphasizing the need for patient-centered assessment and individualized care.
Responsiveness and Minimal Clinically Important Difference (MCID)
Responsiveness to change and the minimal clinically important difference (MCID) are critical aspects of the ESAS tool for palliative care, determining its utility in monitoring treatment effects and patient progress. Hui et al. conducted a multicenter study to specifically identify the MCID for each of the 10 ESAS symptoms. This study, involving 796 cancer patients across six centers, assessed symptom intensity at two clinic visits approximately three weeks apart. Patients also provided a global assessment of change for each symptom, which served as an anchor for MCID determination. The results indicated that ESAS effectively discriminated symptom changes, with area under the receiver-operating characteristic curves ranging from 0.70 to 0.87. Remarkably, a change of just 1 point on the NRS was found to be the optimal cutoff for both symptom improvement and deterioration across all 10 symptoms. This finding, consistently supported by other analytical approaches within the same dataset and a retrospective analysis, highlights the sensitivity of ESAS to even small but clinically meaningful changes in patient symptoms.
Table 2. Minimal Clinically Important Differences for Edmonton Symptom Assessment System (ESAS) Individual Items and Total Scores
| Improvement | Deterioration | |
|---|---|---|
| Symptom | Optimal Cutoff* | Sensitivity |
| Pain | ≥+1 | 0.727 |
| Fatigue | ≥+1 | 0.727 |
| Nausea | ≥+1 | 0.593 |
| Depression | ≥+1 | 0.639 |
| Anxiety | ≥+1 | 0.681 |
| Drowsiness | ≥+1 | 0.599 |
| Appetite | ≥+1 | 0.673 |
| Well being | ≥+1 | 0.664 |
| Dyspnea | ≥+1 | 0.658 |
| Sleep | ≥+1 | 0.728 |
| Physical score† | ≥+3 | 0.630 |
| Emotional score‡ | ≥+2 | 0.585 |
| Total symptom distress scoreψ | ≥+3 | 0.683 |
Minimal Clinically Important Differences for ESAS highlight the tool’s sensitivity in detecting meaningful changes in symptom scores, crucial for monitoring palliative care ESAS tool effectiveness.
ESAS Physical, Emotional and Total Symptom Distress Scores
To provide a more holistic assessment of symptom burden, researchers have proposed composite scores derived from the ESAS tool in palliative care. Building upon the original symptom distress score (SDS), which summed the initial 8 VAS items, and considering the evolution of ESAS, particularly the consistent inclusion of physical and emotional symptoms, the ESAS physical score, emotional score, and total symptom distress score were developed. The ESAS physical score is calculated by summing the scores of six physical symptoms (pain, tiredness, nausea, drowsiness, appetite, and dyspnea), providing a score range of 0–60. The ESAS emotional score is derived from the sum of two emotional symptoms (depression and anxiety), with a range of 0–20. The ESAS total symptom distress score is then calculated by adding the physical score, emotional score, and the well-being item, resulting in a total score range of 0–90. These composite scores are supported by cluster analysis, which has shown that ESAS physical and emotional symptoms tend to group separately. Furthermore, studies have indicated that higher ESAS physical and total symptom distress scores are associated with poorer prognosis, including shortened survival, emphasizing the clinical relevance of these summary measures in palliative care. Recent research has also established MCID cutoffs for these composite scores, providing benchmarks for clinically meaningful improvement and deterioration in overall symptom burden.
Current Applications of ESAS in Palliative Care
The efficiency and systematic nature of the ESAS tool in quantifying multiple symptoms have revolutionized symptom assessment in both clinical and research settings. Its widespread adoption is a testament to its practicality and value in various healthcare disciplines. ESAS offers several advantages, including its ease of administration, interpretation, and reporting, making it a pragmatic patient-centered tool. The simultaneous assessment of 10 symptoms allows for the identification of symptom clusters, providing a more nuanced understanding of patient experiences. Its rapid completion time makes it feasible for routine use in busy clinical environments. The extensive global utilization of ESAS facilitates benchmarking and comparative studies across different populations and healthcare systems. Furthermore, ESAS possesses strong face validity and has been rigorously psychometrically validated. Its availability in over 20 languages and the established MCID values enhance its applicability and clinical utility. However, ESAS also has limitations. It primarily assesses symptom intensity in a unidimensional manner, and the existence of different versions with varying time anchors and items can complicate data comparison. Validation studies in non-cancer populations are relatively limited, and some items, like “well-being,” may lack precise definition. Despite these limitations, ESAS remains a cornerstone in symptom management.
Table 3. Strengths and Limitations of the Edmonton Symptom Assessment Scale
| Strengths | Limitations |
|---|---|
| – Pragmatic patient-centered symptom assessment tool that is easy to administer, interpret and report | – Unidimensional scales that assess only symptom intensity |
| – The assessment of 10 symptoms at the same time allows for symptom clusters to be identified | – Different versions of ESAS are currently used with different time anchors and number of items, making it sometimes difficult to compare or combine results |
| – Can be completed rapidly (<5 minutes) | – Few validation studies in non-cancer populations |
| – Currently used by many clinical and research groups worldwide, allowing for benchmarking | – Some items (e.g. well being) are not well defined |
| – Face validity | |
| – Psychometrically validated by multiple groups | |
| – Available into >20 languages | |
| – The responsiveness and minimal clinically important differences have been identified | |
| – Available in many different languages | |
| – Free of charge |
Understanding the strengths and limitations of the palliative care ESAS tool is crucial for its effective implementation in clinical practice and research, ensuring optimal patient care and data interpretation.
Currently, the palliative care ESAS tool is widely employed for symptom screening and longitudinal monitoring across various palliative care settings, including inpatient, outpatient, and home care environments. Its applications extend beyond palliative care into other medical specialties such as oncology (medical, radiation, surgical, and gynecological), nephrology, cardiology, pulmonology, hepatology, and hematology, demonstrating its versatility in assessing symptom burden across diverse patient populations.
Clinical Applications: Symptom Screening in Palliative Care Practice
In clinical practice, the ESAS tool in palliative care is primarily utilized for systematic symptom screening to identify and address patients’ unmet needs. Cancer Care Ontario (CCO) has integrated ESAS into its province-wide Palliative Care Integration Project (PCIP) since 2006. In this large-scale initiative, patients at ambulatory clinics across 14 Regional Cancer Centers routinely complete ESAS to rate their symptom intensity. Data collection is predominantly electronic, using Interactive Symptom Assessment and Collection (ISAAC) with touch-screen kiosks. By 2014, this project had amassed over 2 million symptom data points from 280,000 patients, achieving a remarkable symptom screening rate and processing over 28,000 patient assessments monthly. Patient feedback has been overwhelmingly positive, with a survey of 3660 patients in Ontario revealing that 92% agreed that ESAS was valuable in helping their healthcare team understand their symptoms and severity. This large-scale implementation highlights the feasibility and acceptance of routine ESAS screening in enhancing palliative care delivery.
The effectiveness of routine symptom data collection relies heavily on clinician engagement and the implementation of appropriate action plans. Surveys of physicians and oncology professionals in Ontario indicate that a majority view ESAS as a helpful starting point for symptom assessment and regularly review patient scores. However, chart audits have revealed a gap between symptom screening and clinical action, with documented interventions for moderate-to-severe symptoms being less frequent than expected. This underscores the necessity for strengthening the link between symptom screening and downstream clinical actions through clinician education, resource allocation, and established care pathways. The integration of ESAS as an automatic trigger for palliative care referral is a promising strategy to bridge this gap, ensuring timely and appropriate interventions for patients with significant symptom burden.
Figure 2. Use of ESAS to Trigger Palliative Care Referral.
Effective utilization of the palliative care ESAS tool requires clinician endorsement and action plans, as illustrated in this model for triggering palliative care referrals based on severe symptom distress.
Clinical Applications: Longitudinal Symptom Monitoring with ESAS
Given the fluctuating nature of symptoms, longitudinal symptom monitoring is crucial in palliative care. The ESAS tool is ideally suited for this purpose, enabling healthcare providers to track symptom changes over time by administering it at each clinic visit. A study involving 1612 cancer patients in an outpatient palliative care clinic demonstrated the value of longitudinal ESAS monitoring. The study revealed that symptom intensity trends differed based on baseline symptom severity. Patients with initially low symptom intensity tended to experience symptom worsening over time, while those with moderate-to-severe symptoms often showed improvement. Overall, a significant proportion of patients with moderate-to-severe symptoms reported improvement, highlighting the dynamic nature of symptom experience and the importance of ongoing assessment. This study underscores the necessity of baseline symptom assessment even in patients with low initial symptom burden, as they are likely to develop concerns in the future. Longitudinal ESAS data provides valuable insights into symptom trajectories and the effectiveness of symptom management strategies, facilitating personalized and adaptive care.
Research Applications: Symptom Trajectory, Clusters, and Modulators
The ESAS tool has significantly advanced symptom research, contributing to a deeper understanding of symptom trajectory, symptom clusters, symptom modulators, and the effectiveness of symptom control interventions. A bibliometric analysis of ESAS publications from 1991 to 2006 documented its rapid integration into global research, particularly in general medicine and oncology. The systematic and longitudinal symptom data collected through ESAS has enabled researchers to explore various dimensions of symptom experience.
The extensive ESAS dataset in Ontario, encompassing over 4 million scores, has provided unique insights into symptom trajectories in advanced illness. Studies analyzing this data have revealed that certain symptoms, such as fatigue, appetite, drowsiness, shortness of breath, and well-being, tend to worsen in the last six months of life, while others, like nausea, depression, anxiety, and pain, may remain relatively stable. Recent research employing Markov Multistate Models has further elucidated symptom trajectories, confirming the rapid deterioration of fatigue and well-being over time in cancer patients. These findings contribute to a more nuanced understanding of symptom progression and inform anticipatory palliative care planning.
The ESAS tool’s capacity to assess multiple symptoms simultaneously has been instrumental in symptom cluster research. Symptom clusters, groups of related symptoms occurring together, are increasingly recognized as significant clinical entities. Studies using ESAS have identified common symptom clusters in palliative care settings, such as physical and emotional symptom clusters. Research employing various statistical approaches has consistently identified clusters of depression and anxiety, as well as clusters of fatigue, drowsiness, and dyspnea in advanced cancer patients. Variations in identified symptom clusters across studies likely reflect differences in patient populations, statistical methods, and ESAS versions used, highlighting the evolving nature of symptom cluster research. More recently, ESAS has been applied to investigate symptom clusters in other conditions, such as advanced heart failure, expanding its research utility beyond oncology.
Furthermore, the ESAS tool has facilitated the identification of symptom modulators—factors that consistently influence symptom expression. Studies examining ESAS data in conjunction with patient characteristics have revealed that factors like a history of alcoholism, smoking, spiritual distress, depression, and anxiety are associated with increased symptom burden across multiple ESAS items. These insights into symptom modulators have significant implications for symptom management, suggesting that addressing underlying modulators, such as emotional and spiritual distress, is crucial for effective symptom control, potentially more so than solely focusing on escalating medication dosages for individual symptoms.
Research Applications: Assessing the Effect of Various Symptom Control Interventions
The ESAS tool has become a valuable outcome measure in research evaluating symptom control interventions. Its ability to simultaneously assess multiple symptoms makes it ideal for capturing the broader impact of interventions, as treating one symptom may influence others. ESAS has been incorporated into numerous observational studies, open-label trials, and randomized controlled trials assessing a wide range of interventions, from pharmacological treatments to non-pharmacological approaches. For example, ESAS has been used to evaluate the effect of dexamethasone on cancer-related fatigue and dyspnea, demonstrating its sensitivity in detecting treatment-related symptom changes. Studies comparing specialty palliative care to usual oncologic care have also utilized ESAS total scores as an outcome measure, revealing that timely palliative care involvement can lead to symptom improvement, while symptoms may worsen with usual care alone. These research applications underscore the utility of ESAS in rigorously evaluating the effectiveness of symptom management strategies and advancing evidence-based palliative care.
The Future of ESAS in Palliative Care
As the ESAS tool continues to gain wider acceptance and application across healthcare settings, ongoing efforts are focused on further enhancing its clinical and research utility. Key areas of future development include standardization, integration with electronic health records, use as a trigger for clinical actions, and incorporation of personalized symptom goals.
Standardization and Continued Validation of ESAS
Addressing the limitations of the ESAS tool, particularly the variability in versions and item definitions, is crucial for future standardization. Standardizing item descriptions and the overall layout of ESAS would facilitate data pooling and comparison across different studies and clinical settings. While both “ESAS now” and “ESAS 24 hour” have their specific applications, clearly defining and consistently reporting the time frame anchor used is essential. Further standardization efforts should also focus on optimizing ESAS administration to enhance accuracy and reliability. Drawing parallels with the broader field of palliative care, where precise definitions are often needed, refining the conceptual clarity of certain ESAS items, such as “depression” and “well-being,” would strengthen its construct validity. Comparative studies evaluating ESAS against other patient-reported outcome measures would also contribute to a more comprehensive understanding of its strengths and limitations and guide its optimal application in palliative care.
Integration of ESAS into Electronic Health Records
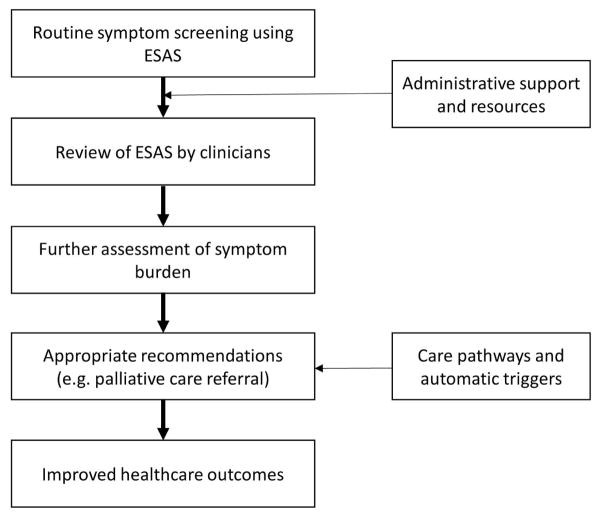
Leveraging information technology to integrate the palliative care ESAS tool into electronic health records (EHRs) holds immense potential. Experiences from initiatives like Cancer Care Ontario’s electronic ESAS collection via kiosks and studies utilizing mobile devices and computers demonstrate the feasibility and benefits of electronic data capture. Electronic ESAS administration offers advantages such as reduced missing data, ease of remote completion, potential for computerized adaptive testing, rapid data access, automated scoring and display, and the ability to incorporate patient alerts and clinical triggers. A cluster randomized controlled trial has shown that providing oncologists with immediate access to electronic ESAS data leads to clinically significant symptom improvement. Despite the upfront costs and implementation challenges associated with EHR integration, the long-term benefits for data management, clinical workflow, and patient care are substantial. Visual display of ESAS data, using bar graphs and symptom expression arrays (Figure 3), can further enhance data interpretation and clinical decision-making.
Figure 3. ESAS Displays.
Visual ESAS displays, such as these graphical representations and symptom expression arrays, enhance interpretation and clinical utility of the palliative care ESAS tool data, facilitating better symptom management.
Use of ESAS to Trigger Clinical Actions and Palliative Care Referrals
The ESAS tool is increasingly recognized for its potential to trigger specific clinical actions, including palliative care referrals. Distress screening is now mandated by accreditation bodies like the American College of Surgeons Commission on Cancer, and ESAS is a recommended tool for this purpose. Systematic reviews of referral criteria for outpatient palliative care have identified symptom distress, often assessed by ESAS, as a common trigger. International expert consensus has established severe physical and emotional distress, defined by ESAS NRS scores ≥7/10, as major criteria for palliative care referral. While these cutoffs may need to be adapted to local contexts and resource availability, the use of ESAS to automatically trigger referrals can ensure timely access to specialist palliative care for patients with high symptom burden. It is important to emphasize that automatic referral triggers should complement, not replace, clinical judgment. Future research should evaluate the impact of ESAS-triggered referrals on patient outcomes and healthcare utilization, as well as patient and clinician perceptions of this approach. Beyond palliative care referrals, ESAS can also guide triage decisions for other clinical actions, such as determining the intensity of home-based palliative care visits, optimizing resource allocation based on patient symptom burden.
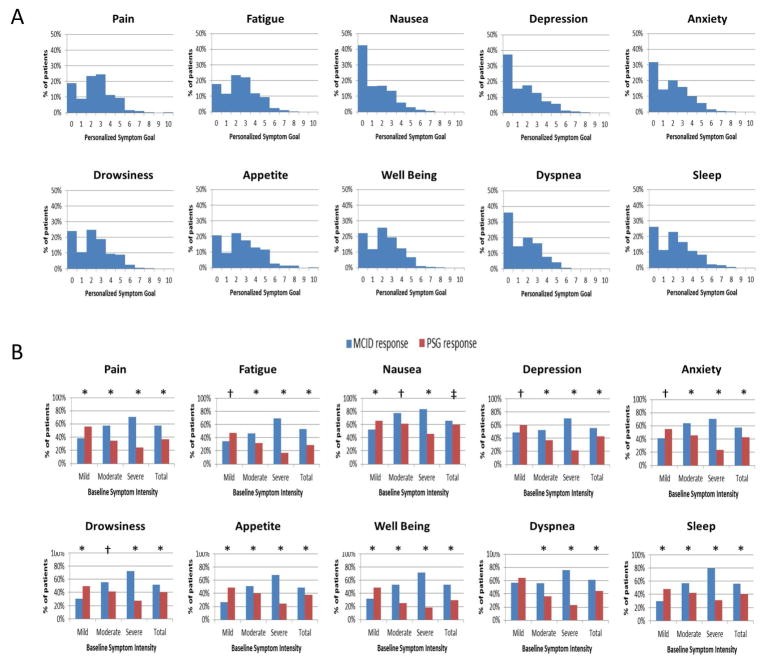
Personalized Symptom Goals with ESAS
Personalized symptom goals (PSGs) represent an innovative approach to enhance the patient-centeredness of the ESAS tool in palliative care. Recognizing that individual patients may interpret NRS scores differently and have varying levels of acceptable symptom burden, PSGs involve asking patients to define their comfortable symptom level on the 0–10 scale. Studies have shown that most patients set PSGs at a score of 3 or less for each ESAS symptom. Integrating PSGs into ESAS assessment allows clinicians to tailor treatment targets to individual patient preferences and expectations. Research has indicated that using PSG achievement as a response criterion provides a more patient-centered measure of treatment success compared to MCID criteria alone. PSGs can be incorporated into both clinical practice and research studies to personalize symptom management and enhance patient-reported outcomes.
Figure 4. Symptom Response Criteria.
Personalized Symptom Goals (PSGs) enhance the patient-centered approach of the palliative care ESAS tool, as illustrated in this distribution of PSGs and comparison of response rates by different criteria.
Conclusion
In summary, the ESAS tool has evolved over the past 25 years into a cornerstone of symptom assessment in palliative care, oncology, and beyond. Its psychometric validation, multilingual availability, and ease of use have facilitated its widespread adoption in both clinical practice and research. ESAS has transformed symptom assessment by enabling rapid, systematic, and multidimensional evaluation of patient-reported symptoms. It has contributed significantly to our understanding of symptom prevalence, trajectories, clusters, modulators, and the effectiveness of symptom control interventions. Ongoing efforts to standardize ESAS, integrate it into EHRs, utilize it to trigger clinical actions, and incorporate personalized symptom goals promise to further enhance its value in optimizing symptom management and improving patient outcomes in palliative care and related fields. As research and technology continue to advance, the ESAS tool will undoubtedly remain a vital instrument in the pursuit of effective and patient-centered symptom management.
