The integrity of our genetic code is constantly challenged by environmental factors and cellular processes. To safeguard against these threats, our cells have developed intricate DNA damage response (DDR) mechanisms. Recent research highlights the critical role of long non-coding RNAs (lncRNAs) in orchestrating these responses. This article delves into the function of a specific lncRNA called ANRIL and its intricate relationship with the transcription factor E2F1 in the context of DNA damage, with a focus on the Coding Atm pathway’s involvement.
The Intricate Dance of DNA Repair
Maintaining the integrity of our DNA is paramount for cell survival and function. Double-strand breaks, a particularly severe form of DNA damage, trigger a complex signaling cascade orchestrated by the coding ATM (Ataxia Telangiectasia Mutated) protein kinase. This kinase initiates a series of events that ultimately lead to DNA repair, cell cycle arrest, or programmed cell death. Within this complex network, lncRNAs are emerging as critical regulators.
ANRIL: A Key Player in the DNA Damage Response
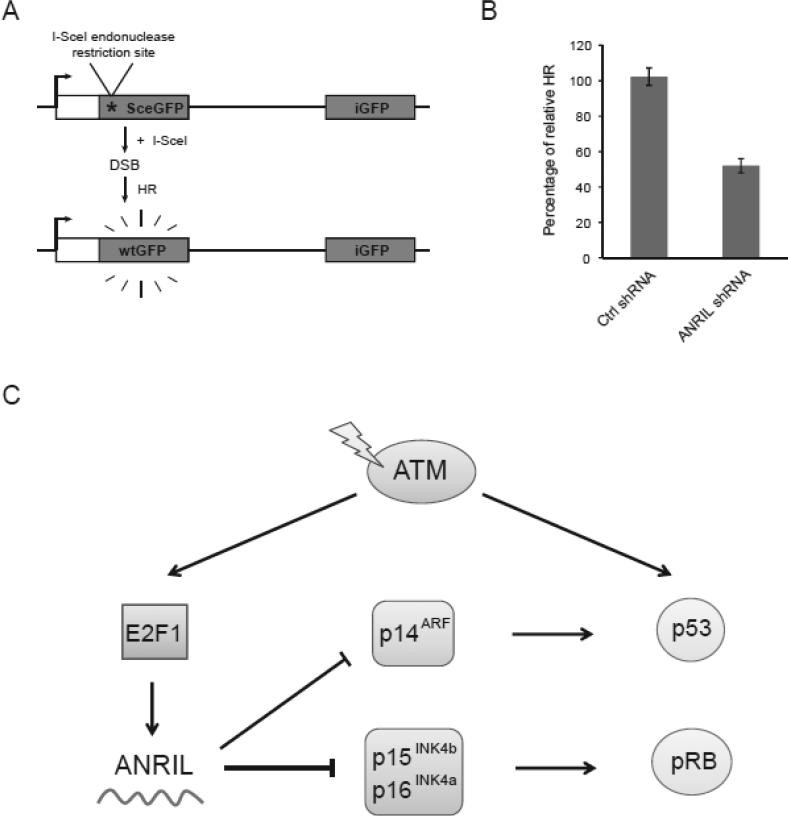
ANRIL, also known as CDKN2B-AS1, is a lncRNA transcribed from the INK4 locus, a region frequently implicated in cancer development. This locus harbors genes encoding tumor suppressor proteins like p15, p16, and p14ARF, which regulate cell cycle progression and apoptosis. ANRIL’s role in modulating the expression of these genes positions it as a central regulator of cellular responses to DNA damage.
E2F1: The Transcriptional Activator of ANRIL
E2F1, a transcription factor known for its role in cell cycle control, has been identified as a crucial regulator of ANRIL expression. Following DNA damage, coding ATM activates E2F1. This activated E2F1 then binds to the ANRIL promoter, initiating its transcription. This ATM-E2F1-ANRIL axis forms a critical regulatory loop in the DDR.
ANRIL’s Impact on Cell Fate
Increased ANRIL levels, driven by E2F1, lead to the suppression of INK4 gene expression. This downregulation of tumor suppressor proteins allows cells to progress through the cell cycle and potentially escape the growth-inhibitory effects of DNA damage. This intricate balance between DNA repair and cell cycle progression is crucial for maintaining genomic stability. Disruptions in this balance, such as aberrant ANRIL expression, can contribute to cancer development.
Conclusion: Implications for Cancer and Beyond
Understanding the intricate interplay between coding ATM, E2F1, and ANRIL provides crucial insights into the complex mechanisms governing the DNA damage response. Dysregulation of this pathway, particularly alterations in ANRIL expression, can disrupt cellular homeostasis and contribute to tumorigenesis. Further research into this critical regulatory axis holds promise for developing novel therapeutic strategies for cancer and other diseases associated with genomic instability. The precise mechanisms by which ANRIL modulates INK4 gene expression and the broader implications of this regulatory network in various physiological and pathological contexts warrant further investigation.