Background
It is widely recognized that patient admission to high-acuity Intensive Care Units (ICUs) correlates with improved patient outcomes compared to those in low-acuity settings. However, the precise mechanisms driving these superior results remain an area of ongoing investigation.
Research Question
This study was designed to explore whether high-acuity ICUs are more effective in implementing evidence-based processes of care, which are known to contribute to better clinical outcomes. This investigation delves into the potential role of a Critical Care Patient Acuity Tool in facilitating these improved processes.
Study Design and Methods
This retrospective cohort study analyzed data from adult ICU patients admitted to 322 ICUs across 199 hospitals, sourced from the Philips ICU telemedicine database between 2010 and 2015. The key factor examined was ICU acuity, determined by the mean Acute Physiology and Chronic Health Evaluation IVa (APACHE IVa) score of all patients admitted within a calendar year. ICUs were then categorized into quartiles based on their acuity levels. Multivariable logistic regression was employed to assess the relationship between ICU acuity and adherence to established evidence-based protocols, specifically Venous Thromboembolism (VTE) and stress ulcer prophylaxis, as well as the avoidance of potentially harmful events such as hypoglycemia, sustained hyperglycemia, and liberal red blood cell (RBC) transfusion practices (defined as transfusions for non-bleeding patients with hemoglobin ≥ 7 g/dL).
Results
The study encompassed 1,058,510 ICU admissions. Adherence to both VTE and stress ulcer prophylaxis was consistently high across all ICU acuity levels. However, after adjusting for confounding variables, patients in low-acuity ICUs were significantly more likely to experience adverse events compared to those in the highest acuity ICUs. These events included hypoglycemia (adjusted Odds Ratio [aOR], 1.12; 95% Confidence Interval [CI], 1.04-1.19), sustained hyperglycemia (aOR, 1.07; 95% CI, 1.04-1.10), and liberal transfusion practices (aOR, 1.55; 95% CI, 1.33-1.82).
Interpretation
The findings indicate a strong association between high-acuity ICUs and enhanced adherence to several key evidence-based practices. This superior adherence may serve as an indicator of high-quality care and suggests that critical care patient acuity tools, like APACHE IVa, play an indirect role in improving care processes. Future research should focus on understanding the organizational strategies employed by high-acuity ICUs to identify specific, actionable targets for improving critical care quality across all ICU acuity levels.
Key Words: critical care, evidence-based medicine, guidelines, ICU, patient safety, critical care patient acuity tool
Abbreviation: APACHE, Acute Physiology and Chronic Health Evaluation
FOR EDITORIAL COMMENT, SEE PAGE 442
Clinical outcomes for patients in ICUs across the United States show considerable variation.1 This variability can be attributed to several factors, including differences in patient case-mix, ICU volume, staffing models, and organizational structures within ICUs.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 ICU acuity, which reflects the average severity of illness of patients within a given ICU, emerges as another significant ICU-level determinant of patient outcomes. This factor operates independently of ICU volume or individual patient acuity.10 Specifically, ICUs that primarily treat patients with higher average illness severity (high-acuity ICUs) have been associated with reduced ICU and hospital lengths of stay and improved mortality rates among patients at lower risk of death, in a dose-dependent manner, when compared to ICUs caring for less severely ill patients (low-acuity ICUs). Identifying the underlying mechanisms that enable high-acuity ICUs to achieve better patient outcomes is crucial for enhancing the quality of care for all ICU patients, regardless of their risk profiles or the type of hospital setting. This understanding can be significantly aided by the effective use of a critical care patient acuity tool to benchmark and improve care standards.
One plausible explanation for these observed differences in outcomes is that high-acuity ICUs excel at implementing and standardizing evidence-based processes of care, which are directly linked to improved clinical outcomes. Processes of care are particularly valuable for quality improvement initiatives because they are largely controllable by clinicians, represent specific, measurable targets for improvement within defined patient groups, and are generally more actionable than broader outcome measures or organizational structures.1,11, 12, 13 However, the adoption and consistent application of evidence-based processes of care across different ICUs remains inconsistent and incomplete.14 Suboptimal adherence to these established processes can be a red flag for potentially lower quality care, which in turn can lead to poorer patient outcomes.15 A reliable critical care patient acuity tool can help identify areas where process improvements are most needed, particularly in relation to patient severity.
Therefore, this study aimed to investigate the relationship between ICU acuity and the performance in five key evidence-based processes of care in ICU patients within hospitals participating in a large ICU telemedicine program in the United States. The study focused on processes of care that are routinely and reliably captured by the Philips ICU telemedicine system, as this system is used to monitor hospital performance and promote continuous quality improvement among its clients. These processes include: (1) adherence to VTE prophylaxis; (2) adherence to stress ulcer prophylaxis; (3) avoidance of hypoglycemia; (4) avoidance of sustained hyperglycemia; and (5) avoidance of liberal transfusion practices.16, 17, 18, 19, 20, 21, 22, 23, 24, 25 The central hypothesis was that high-acuity ICUs would demonstrate greater adherence to these evidence-based processes compared to low-acuity ICUs. The study implicitly explores the utility of critical care patient acuity tools in predicting and potentially driving better adherence to these practices.
Materials and Methods
This retrospective cohort study leveraged the Philips eICU Research Institute data repository, which compiles clinical and administrative data from over 320 hospitals across the United States.26, 27, 28 The ICUs included in this program are geographically diverse, encompassing critical access, community, and referral hospitals. They also serve communities of varying sizes, with 14% serving communities of over 1 million people, 51% serving communities of 100,000 to 999,999, and 34% serving smaller communities. Data from these ICUs are centrally aggregated in the Philips eICU Research Institute data repository, providing granular, robust, and standardized data on admissions, discharges, demographics, laboratory results, and medications for all patients admitted to participating ICUs.10,26,27 This rich dataset allows for detailed analysis of ICU performance and the potential impact of critical care patient acuity tools on care delivery.
Study Population
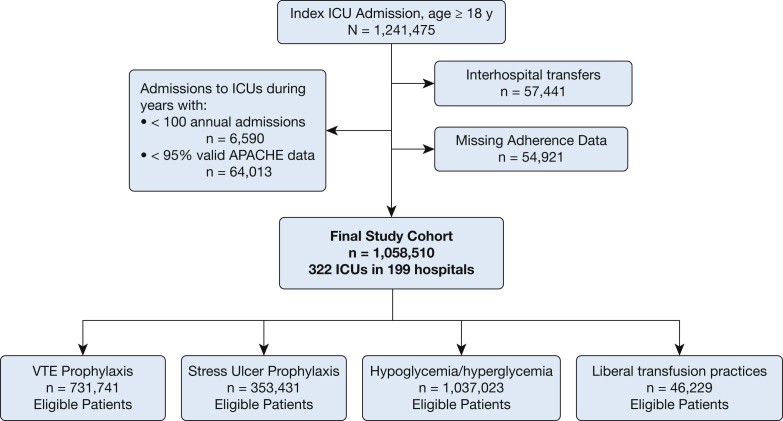
The study cohort comprised patients aged 18 years or older who were admitted to ICUs participating in the Philips ICU telemedicine program between 2010 and 2015. Figure 1 visually outlines the patient selection process. Patients with incomplete or invalid data required for calculating an APACHE IVa score were excluded. Patients transferred to or from other facilities were also excluded to ensure a complete understanding of their hospital course and outcomes. For patients with multiple ICU admissions during the study period, only the first admission was included to maintain independence of outcomes. Admissions to ICUs with consistently low patient volumes (defined as < 50 admissions per year) were also excluded to ensure the reliability of acuity assessments.
Figure 1.
Combined ICU and patient-level exclusion criteria. ACUTE = Acute Physiology and Chronic Health Evaluation. Alt text: Flowchart illustrating patient selection process for ICU study, detailing inclusion and exclusion criteria based on age, ICU admission data completeness, transfers, readmissions, and ICU volume, leading to the final study cohort.
Exposure and Outcome Variables
The primary exposure variable was ICU acuity, defined by the mean APACHE IVa score of all patients admitted to an ICU within a calendar year.10 APACHE IVa is a validated severity-of-illness scoring system used in ICUs. It incorporates physiological variables, diagnoses, and comorbidities to predict patient outcomes, including ICU and hospital mortality and length of stay.29 After confirming a near-normal distribution of ICU acuity scores, ICUs were categorized into quartiles representing low, medium, high, and highest acuity levels for each year of the study. This categorization facilitated comparisons between ICUs and enhanced the interpretability of the results. It is important to note that ICUs could shift between acuity categories from year to year based on their relative mean APACHE IVa scores. Teaching hospitals were identified based on their membership in the Council of Teaching Hospitals and Health Systems.30 APACHE IVa, as a critical care patient acuity tool, was central to defining the exposure in this study.
The primary outcomes measured were the appropriate administration of VTE or stress ulcer prophylaxis to eligible patients and the avoidance of hypoglycemic events, sustained hyperglycemia, and liberal transfusion practices. These patient-level outcomes are routinely monitored by the Philips ICU telemedicine program as key performance indicators, reflecting adherence to evidence-based processes of care.26 Patients admitted to the ICU for more than 24 hours were considered eligible for VTE prophylaxis unless they were already receiving full-dose anticoagulation or if a clinician explicitly documented a contraindication. Adherence to VTE prophylaxis was defined as documented administration of either pharmacologic (e.g., unfractionated heparin, low-molecular-weight heparin, warfarin, or other anticoagulants) or mechanical prophylaxis (i.e., intermittent compression device) within 48 hours of ICU admission for eligible patients.
Eligibility for stress ulcer prophylaxis was determined by the presence of active medical problems such as head injury, serious burns, or coagulopathy, or if the patient was mechanically ventilated for more than 24 hours. Adherence to stress ulcer prophylaxis was defined as documented administration of pharmacologic prophylaxis (i.e., antacids, histamine2-receptor antagonists, proton pump inhibitors, sucralfate) within 24 hours of identifying a risk factor for stress ulcers.
Hypoglycemia was defined by the Philips ICU telemedicine program as at least one documented glucose value
Analysis
Each analysis was restricted to patients who met the eligibility criteria for the specific intervention being assessed. For the blood transfusion outcome, only patients who received at least one RBC transfusion during their ICU stay were included. Descriptive statistics were used to summarize all variables. Unadjusted differences between ICU acuity levels were assessed using χ2 tests for categorical variables and Wilcoxon rank sum tests for continuous variables, as appropriate. Table 1 presents the results of these descriptive statistical analyses.
Table 1.
Characteristics and Unadjusted Outcomes of Patients Stratified According to ICU Acuity
| Variable | Low-Acuity ICUs (n = 224,294) | Medium-Acuity ICUS (n = 237,128) | High-Acuity ICUs (n = 260,372) | Highest Acuity ICUs (n = 336,716) |
|---|---|---|---|---|
| Age, y | 62.8 ± 17.1 | 63.8 ± 16.7 | 63.4 ± 17.0 | 63.5 ± 16.9 |
| Male sex | 120,002 (53.5) | 128,884 (54.4) | 139,807 (53.7) | 180,612 (53.6) |
| Race | ||||
| White | 170,591 (76.1) | 179,034 (75.5) | 202,091 (77.6) | 251,840 (74.8) |
| Black | 24,442 (10.9) | 28,747 (12.1) | 25,948 (10.0) | 40,643 (12.1) |
| Other | 29,261 (13.0) | 29,347 (12.4) | 32,333 (12.4) | 44,233 (13.1) |
| APACHE IVa score | 47.8 ± 21.8 | 52.4 ± 22.7 | 56.1 ± 24.7 | 62.8 ± 27.8 |
| Admission source | ||||
| ED | 121,960 (54.4) | 127,231 (53.7) | 142,233 (54.6) | 183,399 (54.5) |
| Operating room | 34,083 (15.2) | 44,405 (18.7) | 40,496 (15.6) | 43,639 (13.0) |
| Ward transfer | 28,105 (12.5) | 33,127 (14.0) | 40,475 (15.5) | 57,411 (17.1) |
| Direct admission | 21,524 (9.6) | 16,110 (6.8) | 18,818 (7.2) | 26,122 (7.8) |
| Other | 18,622 (8.3) | 16,255 (6.9) | 18,350 (7.0) | 26,145 (7.8) |
| Admitting diagnosis | ||||
| Cardiac | 55,795 (24.9) | 74,383 (31.4) | 68,566 (26.3) | 74,456 (22.1) |
| Diabetic ketoacidosis | 4,126 (1.8) | 4,992 (2.1) | 5,689 (2.2) | 7,969 (2.4) |
| GI bleeding | 9,876 (4.4) | 12,646 (5.3) | 15,349 (5.9) | 23,062 (6.8) |
| Neurologic | 42,292 (18.9) | 24,772 (10.4) | 27,658 (10.6) | 30,777 (9.1) |
| Overdose | 6,025 (2.7) | 6,742 (2.8) | 8,004 (3.1) | 10,280 (3.1) |
| Respiratory | 5,246 (2.3) | 7,282 (3.1) | 7,473 (2.9) | 10,167 (3.0) |
| Sepsis | 34,884 (15.6) | 44,781 (18.9) | 56,180 (21.6) | 95,483 (28.4) |
| Trauma | 16,115 (7.2) | 9,174 (3.9) | 11,833 (4.5) | 12,124 (3.6) |
| Other | 49,935 (22.3) | 52,356 (22.1) | 59,620 (22.9) | 72,398 (21.5) |
| ICU LOS, d | 2.8 ± 3.5 | 2.9 ± 3.7 | 2.9 ± 3.7 | 3.2 ± 4.1 |
| ICU mortality | 8,493 (3.8) | 10,682 (4.5) | 14,287 (5.5) | 25,367 (7.5) |
| Hospital LOS, d | 6.8 ± 6.5 | 7.4 ± 7.0 | 7.7 ± 7.2 | 8.7 ± 8.2 |
| Hospital mortality | 15,640 (7.0) | 18,648 (7.9) | 22,914 (8.8) | 39,397 (11.7) |
Data are presented as mean ± SD or No. (%). APACHE = Acute Physiology and Chronic Health Evaluation; LOS = length of stay. Alt text: Table 1 displaying patient characteristics and unadjusted outcomes across ICU acuity levels, including age, sex, race, APACHE IVa score, admission source and diagnosis, length of stay, and mortality rates.
Next, patient-level multivariable analyses were conducted using mixed effects logistic regression, with ICU as a random effect to account for the non-independence of data within hospital sites. Hospital discharge year was included as a fixed effect to control for potential confounding due to practice changes over time.31,32 Adjustments were made for pre-selected potential confounders, including severity of illness (APACHE IVa score), year of hospital discharge, ICU type, ICU volume, number of hospital beds, and hospital teaching status. Given that the APACHE IVa score already incorporates patient-level variables such as age, diagnosis, and chronic comorbidities, these were not included as separate covariates in the models. ICU-level covariates were aggregated at the ICU level, while all analyses were performed at the patient level. The use of APACHE IVa as a critical care patient acuity tool was fundamental in adjusting for severity of illness in these analyses.
Several secondary analyses were also planned. First, ICU acuity was analyzed as a continuous variable rather than a categorical one. Second, the cohort was narrowed to include only patients at low risk of mortality, based on prior evidence suggesting that this group benefits most from admission to high-acuity ICUs.10 Low-risk patients were defined as those with an APACHE IVa-predicted hospital mortality of ≤ 3%.10,33 Finally, a post hoc exploratory analysis was performed to test for an interaction effect between ICU acuity and teaching status, aiming to uncover additional organizational factors that might influence adherence to evidence-based practices.
P values
Results
The final study population consisted of 1,058,510 patients admitted to 322 ICUs in 199 hospitals. Table 1 provides a summary of patient characteristics and unadjusted outcomes categorized by ICU acuity levels. Cardiac diagnoses were the most frequent admitting diagnosis, and the emergency department (ED) was the most common admission source across all ICU acuity quartiles. Approximately 60% of the ICUs were mixed medical-surgical units. Hospitals showed considerable variation in bed size and annual patient volume, and most ICUs were in non-teaching hospitals (Table 2).30
Table 2.
Characteristics of ICUs Stratified According to ICU Acuity
| Characteristic | Low-Acuity ICUs (n = 131) | Medium-Acuity ICUs (n = 151) | High-Acuity ICUs (n = 146) | Highest Acuity ICUs (n = 120) |
|---|---|---|---|---|
| ICU type | ||||
| Medical-surgical | 78 (59.5) | 87 (57.6) | 86 (58.9) | 70 (58.3) |
| Specialty/other | 53 (40.5) | 64 (42.4) | 60 (41.1) | 50 (41.7) |
| Hospital bed size | ||||
| 21 (16.0) | 20 (13.2) | 14 (9.6) | 6 (5.0) | |
| 100-249 | 40 (30.5) | 42 (27.8) | 37 (25.3) | 18 (15.0) |
| 250-499 | 19 (14.5) | 25 (16.6) | 33 (22.6) | 36 (30.0) |
| ≥ 500 | 25 (19.1) | 41 (27.2) | 46 (31.5) | 46 (38.3) |
| Missing | 26 (19.8) | 23 (15.2) | 16 (11.0) | 14 (11.7) |
| Teaching hospitals | 20 (15.3) | 24 (15.9) | 30 (20.5) | 33 (27.5) |
Data are presented as No. (%). Counts represent the number of unique ICUs at each acuity level. Because of the way ICU acuity was defined, ICUs could belong to more than one quartile over the study period. Alt text: Table 2 showing characteristics of ICUs by acuity level, including ICU type (medical-surgical vs. specialty/other), hospital bed size categories, and proportion of teaching hospitals.
For VTE and stress ulcer prophylaxis, overall adherence rates were high, with minimal variation across different acuity levels (96.6% of 731,741 eligible patients and 90.5% of 353,431 eligible patients, respectively) (e-Fig 1). Adjusted analyses for these two processes of care similarly showed high adherence rates with no clinically significant differences across ICU acuity levels (e-Table 1, e-Appendix 1). In contrast, potentially harmful events like sustained hyperglycemia and liberal transfusion practices occurred more frequently and exhibited greater variability across ICUs (e-Fig 2). Adjusted analyses for hypoglycemia, sustained hyperglycemia, and liberal transfusion practices revealed that patients admitted to low-, medium-, and high-acuity ICUs were more likely to experience hypoglycemia and sustained hyperglycemia compared to those in the highest acuity ICUs. For transfusion practices, higher ICU acuity was associated with lower odds of liberal transfusion practices in a dose-dependent manner (Table 3).
Table 3.
ICU Acuity and Odds of Experiencing Hypoglycemia, Sustained Hyperglycemia, or Liberal Transfusion Practices
| ICU Acuity | Hypoglycemia (n = 1,037,023a) | Hyperglycemia (n = 1,037,023a) | Transfusion (n = 46,229a) |
|---|---|---|---|
| Highest | Reference | Reference | Reference |
| High | 1.10 (1.05-1.15) | 1.05 (1.03-1.07) | 1.08 (0.98-1.18) |
| Medium | 1.11 (1.05-1.18) | 1.04 (1.02-1.06) | 1.41 (1.25-1.59) |
| Low | 1.12 (1.04-1.19) | 1.07 (1.04-1.10) | 1.55 (1.33-1.82) |
Data are presented as OR (95% CI).
aTotal number of eligible patients for each outcome. Alt text: Table 3 showing odds ratios and confidence intervals for hypoglycemia, hyperglycemia, and liberal transfusion practices across different ICU acuity levels, with the highest acuity group as the reference.
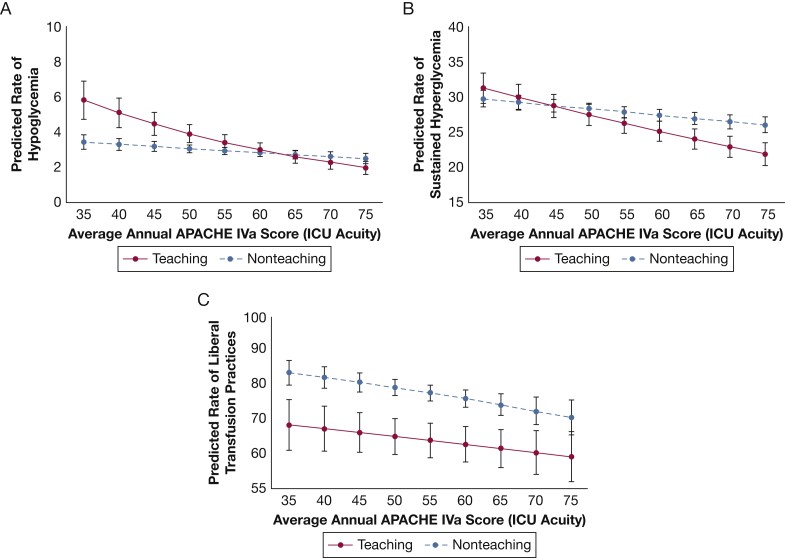
In post hoc exploratory analyses, using ICU acuity as a continuous variable and including an interaction term for ICU acuity and teaching status, it was found that low-acuity teaching hospitals had a higher predicted probability of hypoglycemia and sustained hyperglycemia compared to low-acuity non-teaching hospitals. As ICU acuity increased, the predicted probability of both hypoglycemia and sustained hyperglycemia decreased, and this effect was more pronounced in teaching hospitals (Figs 2A and 2B). Conversely, teaching hospitals showed a lower predicted probability of liberal transfusion practices compared to non-teaching hospitals, regardless of ICU acuity level (Fig 2C). In analyses limited to low-risk patients, adherence to VTE and stress ulcer prophylaxis remained high (195,508 of 204,293 [95.7%] and 41,191 of 50,529 [83.5%] eligible patients, respectively), with no significant differences across acuity levels. Similarly, no significant differences were found across ICU acuity levels for hypoglycemia or sustained hyperglycemia outcomes in this low-risk subgroup. However, for transfusion practices, the pattern observed in the primary analysis persisted, with increasing ICU acuity associated with progressively lower odds of liberal transfusion practices.
Figure 2.
Expected rate of (A) hypoglycemic events, (B) sustained hyperglycemia, and (C) liberal transfusion practices based on ICU acuity as defined by the average APACHE IVa score per ICU year. Each line represents results for teaching hospitals compared with nonteaching hospitals. APACHE IVa scores 70 were collapsed into two groups given the small number of patients in the study cohort with scores beyond these thresholds. See Figure 1 legend for expansion of abbreviation. Alt text: Line graphs illustrating the expected rates of hypoglycemic events, sustained hyperglycemia, and liberal transfusion practices in teaching versus non-teaching hospitals across a spectrum of ICU acuity levels, based on APACHE IVa scores.
Discussion
This retrospective cohort study, conducted across a wide network of ICUs participating in a national ICU telemedicine program, revealed consistently high rates of VTE and stress ulcer prophylaxis with minimal variability among ICUs. Notably, the study demonstrated that higher ICU acuity is associated with a reduced likelihood of potentially harmful events, including hypoglycemia, sustained hyperglycemia, and liberal transfusion practices. This improved performance in evidence-based practices may be a hallmark of high-quality care and could correlate with better adherence to other unmeasured best practices. This offers a potential explanation for previous findings that high-acuity ICUs are linked to improved risk-adjusted patient outcomes, such as reduced length of stay and mortality.10 The effective use of a critical care patient acuity tool like APACHE IVa is implied in the ability of high-acuity ICUs to achieve these better outcomes.
The selection of five evidence-based processes of care was based on a thorough review of existing literature and the availability of data from the Philips eICU Research Institute.17, 18, 19, 20, 21, 22,24,27 While prior research has established links between these process measures and improved patient outcomes, adherence to these measures may also serve as a broader indicator of high-quality care that extends beyond what is directly measured. For instance, a 2008 study by Werner et al.13 indicated that the unquantified aspects of care associated with aspirin administration are substantially more impactful than aspirin’s direct pharmacological effect on mortality in patients with acute myocardial infarction. Similarly, it is reasonable to assume that ICUs with better adherence to evidence-based practices are also likely to excel in other unmeasured aspects of care that influence patient outcomes, such as care coordination and the infrastructure supporting quality improvement initiatives. These findings collectively underscore the importance of using a multifaceted approach to assess ICU quality, incorporating structure, process, and outcome measures. They also highlight the potential to further improve patient outcomes by studying high-performing ICUs and disseminating their best practices. Mixed-methods research approaches focusing on high-performing ICUs could be particularly valuable in identifying currently unmeasured factors contributing to their superior outcomes, with the ultimate goal of broadly implementing these findings across all hospitals.13 The role of a standardized critical care patient acuity tool in facilitating this benchmarking and best practice dissemination should not be underestimated.
The results of this study also build upon existing research on the volume-outcome relationship in critical care. A comprehensive systematic review and meta-analysis previously found that patients at the highest risk of death are most likely to benefit from treatment in high-volume centers.7 However, organizational factors at the ICU and hospital levels were identified as key determinants of this volume-outcome relationship. A 2017 study examining the volume-outcome relation in sepsis demonstrated that certain evidence-based processes of care are more likely to be implemented in high-volume hospitals.34 This study adds to this body of literature by demonstrating a significant association between ICU acuity and adherence to evidence-based processes of care, independent of ICU volume. These findings contribute valuable insights to ongoing discussions about the regionalization of critical care by shedding light on the factors that enable some ICUs to outperform others.
Furthermore, the study’s finding of high adherence to VTE and stress ulcer prophylaxis, with low variability across hospitals within a large telemedicine program, is consistent with a multicenter observational study evaluating the relationship between specific components of ICU telemedicine interventions and patient outcomes. That study found that several components of telemedicine interventions, including timely remote intensivist review, improved adherence to evidence-based practices, reduced alarm response times, and the real-time use of performance measures, were associated with better patient outcomes.35 Improved adherence to evidence-based practices, such as VTE and stress ulcer prophylaxis, may therefore be a crucial mechanism contributing to the potential effectiveness of ICU telemedicine interventions. Telemedicine platforms may also integrate critical care patient acuity tools to further enhance care delivery.
Post hoc exploratory analyses revealed that teaching status significantly influenced the relationship between ICU acuity and the odds of experiencing hypoglycemia, sustained hyperglycemia, and liberal transfusion practices, although in varying patterns. At lower ICU acuity levels, teaching hospitals exhibited higher predicted probabilities of hypoglycemia and sustained hyperglycemia, a pattern that reversed at higher acuity levels. In contrast, teaching hospitals consistently showed lower predicted probabilities of liberal transfusion practices, regardless of ICU acuity. The underlying mechanisms for these observed differences are not yet clear and warrant further investigation. Prior research has shown that the use of standardized protocols for glucose management in the ICU is associated with better glucose control and improved patient outcomes.36 Differences in protocol utilization and the roles of nurses, pharmacists, and trainees in glucose management between teaching and non-teaching hospitals may contribute to the variability in outcomes observed in this study. Similarly, teaching hospitals are more frequently located within academic medical centers,14 which often have dedicated intensivists with specialized training in evidence-based transfusion practices and management of complex ICU diagnoses. Collectively, these findings suggest that high-acuity ICUs may foster organizational environments that are more conducive to implementing systems-level quality improvement interventions, including more efficient data collection and reporting, compared to low-acuity ICUs. Further research is needed to thoroughly understand the organizational approaches of high-acuity ICUs and to identify specific targets for improving critical care quality across all levels of ICU acuity. This includes exploring how critical care patient acuity tools are utilized within these high-performing environments.
This study has several limitations that should be considered. First, while the study cohort included a diverse range of ICUs varying in location, size, type, and teaching status, all participating ICUs were part of an ICU telemedicine program. This program itself is an ICU-level intervention known to enhance adherence to certain evidence-based practices.35,37,38 Second, data on other hospital characteristics and organizational factors, such as the use of checklists, standardized order sets, staffing ratios including clinical pharmacists on rounds and patient-to-nurse ratios, were not available and could not be included in the analyses. Third, the study restricted each analysis to patients eligible for the specific intervention, and both the determination of patient eligibility and the definition of process variables relied on chart documentation, which carries a potential risk of misclassification bias. However, the study’s focus on comparisons between low-acuity and highest acuity ICUs aimed to maximize the differences in exposure variables. Fourth, for patients with only one glucose measurement per day, the daily average glucose value was based on that single measurement, potentially not capturing the full glycemic variability.
Conclusions
In conclusion, this study demonstrated that admission to higher-acuity ICUs is associated with better adherence to evidence-based practices compared to admission to low-acuity ICUs. These findings suggest that high-acuity ICUs may be more effective in implementing and standardizing evidence-based processes of care that are linked to improved patient outcomes. Future research should further investigate the organizational strategies employed by high-acuity ICUs to pinpoint specific targets for enhancing the quality of critical care across all ICU acuity levels and explore the role of critical care patient acuity tools in this process.
Acknowledgments
Author contributions: K. C. V., J. Y. S., and O. B. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. K. C. V., O. B., M. O. H., C. G. S., and M. P. K. contributed to the conception and design of this study. O. B. contributed to data acquisition. K. C. V. and J. Y. S. contributed to the analysis of data. K. C. V., J. Y. S., O. B., M. O. H., C. G. S., D. R. S., and M. P. K. contributed to interpretation of data. All authors have made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; have contributed to drafting the article for important intellectual content; and have provided final approval of the version to be published.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: O. B. is an employee of Philips Healthcare. None declared (K. C. V., J. Y. S., M. O. H., C. G. S., D. R. S., M. P. K.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: This article was reviewed and approved by Craig Lilly, MD; Louis Gidel, MD, PhD; Richard Riker, MD; Leo Celi, MD, MS, MPH; Teresa Rincon, RN, BSN, eCCRN; Theresa Davis, PhD, RN, NE-BC, CHTP; and Michael Waite, MD of the Philips eICU Research Institute Publications Committee. They were not compensated for this review.
Additional information: The e-Appendix, e-Figures and e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Department of Veterans Affairs did not have a role in the conduct of the study; in the collection, management, analysis, or interpretation of data; or in the preparation of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the US Government.
FUNDING/SUPPORT: Dr Vranas is supported by grant 5K12HL133115. Dr Harhay is supported by grants K99HL141678 and R00HL141678. Dr Slatore is supported by resources from the VA Portland Health Care System. Dr Sullivan is supported by grant K07CA190706.
Supplementary Data
e-Online Data
mmc1.pdf (220KB, pdf)
References
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
e-Online Data
mmc1.pdf (220KB, pdf)

